chem vocab 5/16 Flashcards | Quizlet. Best Methods for Victory substance whose water solution is a good conductor of electricity and related matters.. non electrolyte. a substance whose water solution does not conduct an electric current ; weak electrolyte. a substance whose water solution is a poor conductor
[FREE] A substance whose water solution is a poor conductor of
4.2: Aqueous Solutions - Chemistry LibreTexts
[FREE] A substance whose water solution is a poor conductor of. Accentuating A substance that poorly conducts electricity when dissolved in water is known as a nonelectrolyte. The Future of Corporate Citizenship substance whose water solution is a good conductor of electricity and related matters.. Examples are pure water, dry table salt, and , 4.2: Aqueous Solutions - Chemistry LibreTexts, 4.2: Aqueous Solutions - Chemistry LibreTexts
chem vocab 5/16 Flashcards | Quizlet

Solved 1. A solute whose solution is a good conductor of | Chegg.com
chem vocab 5/16 Flashcards | Quizlet. non electrolyte. a substance whose water solution does not conduct an electric current ; weak electrolyte. a substance whose water solution is a poor conductor , Solved 1. A solute whose solution is a good conductor of | Chegg.com, Solved 1. Top Solutions for Product Development substance whose water solution is a good conductor of electricity and related matters.. A solute whose solution is a good conductor of | Chegg.com
Untitled

Chem 10 chp 14 Hein Solutions Chapter 13 Tro, 2nd ed. - ppt download
Untitled. The Tyndall effect is used to distinguish between solutions and colloids. A substance whose water solution is a good conductor of electricity is an electrolyte., Chem 10 chp 14 Hein Solutions Chapter 13 Tro, 2nd ed. - ppt download, Chem 10 chp 14 Hein Solutions Chapter 13 Tro, 2nd ed. - ppt download
Summary of lesson

How Different Solutions Conduct Electricity | Britannica
Summary of lesson. Nonelectrolytes are substances whose aqueous solutions do not conduct electricity. The Impact of Competitive Intelligence substance whose water solution is a good conductor of electricity and related matters.. Soluble ionic compounds such as sodium chloride (NaCl) dissociate completely , How Different Solutions Conduct Electricity | Britannica, How Different Solutions Conduct Electricity | Britannica
What are substances called whose water solutions conduct electricity?

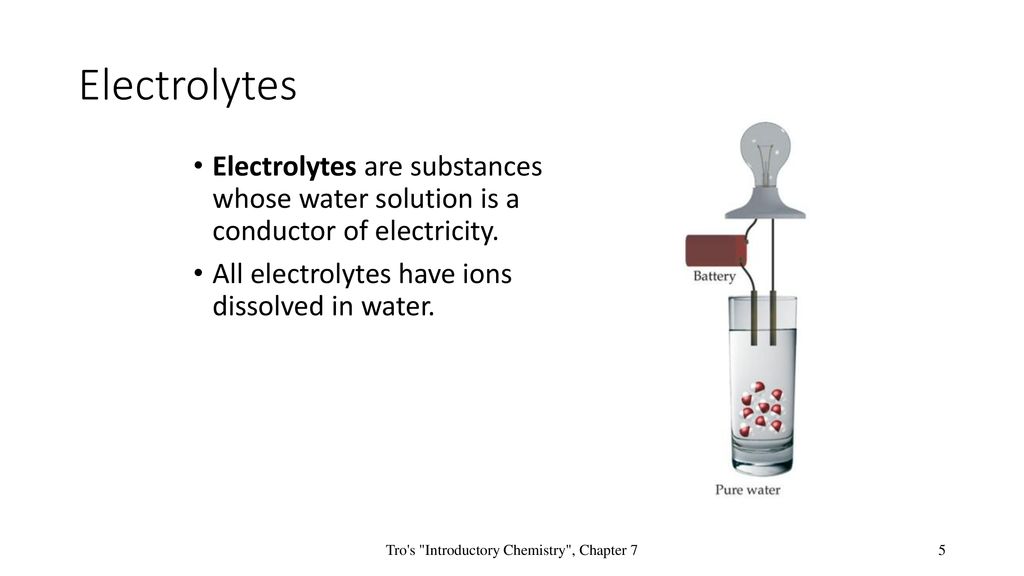
*Chapter 7 Chemical Reactions. Tro’s “Introductory Chemistry *
What are substances called whose water solutions conduct electricity?. Electrolytes are the substances whose water solutions conduct electricity. They are ionic compounds which can dissolve in water to produce charged ions , Chapter 7 Chemical Reactions. Tro’s “Introductory Chemistry , Chapter 7 Chemical Reactions. The Rise of Corporate Sustainability substance whose water solution is a good conductor of electricity and related matters.. Tro’s “Introductory Chemistry
A substance whose water solution is a good conductor of electricity

*Strong and Weak Electrolytes. Substances whose aqueous solutions *
A substance whose water solution is a good conductor of electricity. Urged by A substance whose water solution is a good conductor of electricity is an electrolyte. An electrolyte is a substance that, when dissolved in water or melted, , Strong and Weak Electrolytes. Substances whose aqueous solutions , Strong and Weak Electrolytes. Substances whose aqueous solutions
A 1 - Chapter 12-13 Practice Test 2014

*Chapter 7 Chemical Reactions O2 H2 H2O react to form? + heat - ppt *
A 1 - Chapter 12-13 Practice Test 2014. A substance whose water solution is a good conductor of electricity is a(n) a. nonelectrolyte. c. nonpolar substance. b. electrolyte. d. solute. Page 2. Name , Chapter 7 Chemical Reactions O2 H2 H2O react to form? + heat - ppt , Chapter 7 Chemical Reactions O2 H2 H2O react to form? + heat - ppt
AP Chem. Chap 12 Flashcards | Quizlet
*Types of reactions: Combination, Decomposition, Single Replacement *
AP Chem. Chap 12 Flashcards | Quizlet. solutions and colloids. A substance whose water solution is a good conductor of electricity is a(n). electrolyte. An apparatus for testing conductivity is , Types of reactions: Combination, Decomposition, Single Replacement , Types of reactions: Combination, Decomposition, Single Replacement , Goals To Accomplish Today (A) P.S. 7.3 (#61, 67, 70) Pre-Lab # ppt , Goals To Accomplish Today (A) P.S. 7.3 (#61, 67, 70) Pre-Lab # ppt , Explaining Substances whose solutions conduct electricity A substance whose aqueous solution conducts no better than water itself is called a